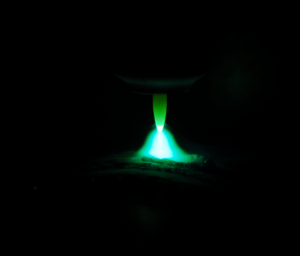
Electrolysis is an important process in chemistry and industry in which an electrical current source is used to trigger a non-spontaneous chemical reaction. Typically, electrolysis is used to break down a substance into its components or to form new compounds.
In an electrolytic cell, two electrodes are immersed in an electrolyte solution consisting of ions. One electrode is called the anode and the other the cathode. When an electrical voltage is applied, the positively charged ions move to the cathode, while the negatively charged ions move to the anode. Redox reactions then take place at the electrodes, leading to the desired transformation.
A well-known example of electrolysis is hydrogen electrolysis, in which water is broken down into oxygen and hydrogen. Oxygen gas is produced at the anode by the oxidation of water, while hydrogen gas is produced at the cathode by the reduction of water.
Electrolysis is used in many industrial processes, including the extraction of metals such as aluminium and sodium, the production of chlorine and caustic soda and the electroplating of metals. It is a versatile process that enables a wide range of applications in various industries.
aixcon has the right power supply for your application. We customise it to your parameters and requirements. Our power supplies for electrolysis are used in coating processes as well as for hydrogen production and are utilised worldwide. Our portfolio already includes power sources from 10kW to 2 MW.