Electrolysis
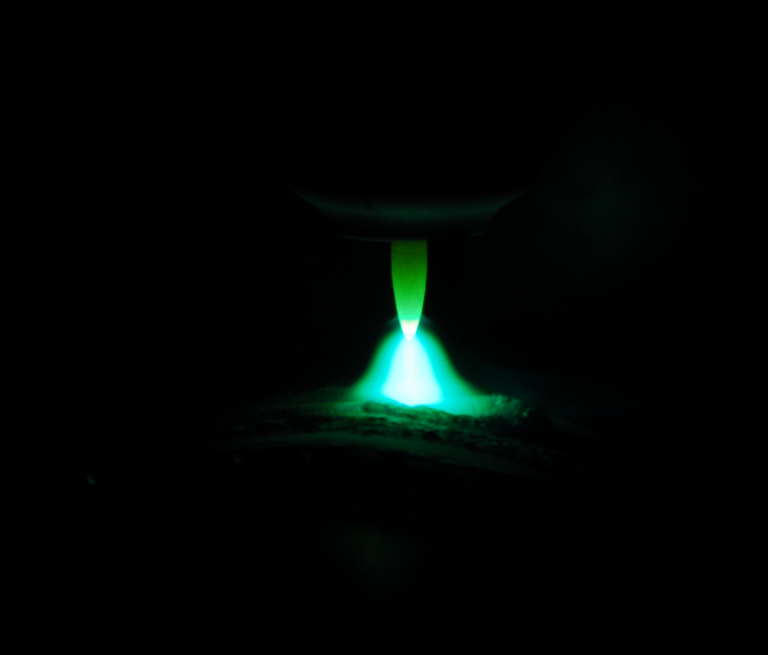
Electrolysis is an important process in chemistry and industry in which an electrical current source is used to trigger a non-spontaneous chemical reaction. Typically, electrolysis is used to break down a substance into its components or to form new compounds. In an electrolytic cell, two electrodes are immersed in an electrolyte solution consisting of ions. One electrode is called the anode and the other the cathode. When an electrical voltage is applied, the positively charged ions move to the cathode, …